UDI Implementation
The U.S. Food and Drug Administration (FDA) has issued a final rule to establish a system to adequately identify devices through distribution and use. This rule requires manufacturers of Class I, II and III medical devices to publish unique device identifiers (UDIs) and product data attributes to the FDA Global UDI database (GUDID). Ecolab’s Class I and Class II medical devices fall under this FDA mandate.
The rule also requires medical device labeling to include a new date format (YYYY-MM-DD) to harmonize with ISO 8601.
Ecolab expects to have its Class II device labeling compliant with the date format by the end of Q2 2016. Publication of the Unique Device Identifiers and product data to the GUDID will be completed by 9/24/2016 for Class II devices and 9/24/2018 for Class I devices.
Ecolab expects to have its Class II device labeling compliant with the date format by the end of Q2 2016. Publication of the Unique Device Identifiers and product data to the GUDID will be completed by 9/24/2016 for Class II devices and 9/24/2018 for Class I devices.

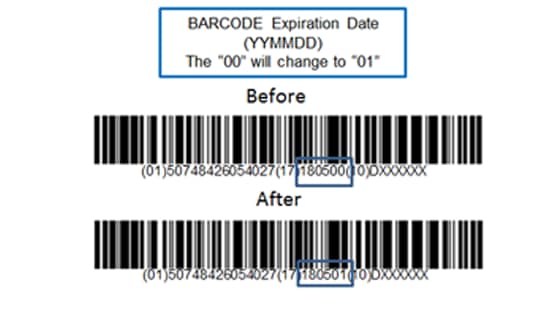
Please Note:
The FDA deadline for publishing UDI and product data for Class II is September 24, 2016 and for Class I is September 24, 2018. For additional information about this process please visit the FDA website via the links we have provided.

