
Bioquell ProteQ
Treat nearly any room or area in your facility with this mobile, scalable system. Ecolab’s Bioquell ProteQ features wireless communication technology, built-in aeration, and the option to add additional aeration capability.
Benefits of Bioquell ProteQ
Bioquell ProteQ is a great choice for everything from small labs to large bioprocessing areas, for routine application to emergency remediation.
Powerful hydrogen peroxide vapor distribution reduces cycle times, and wireless functionality can network multiple units.
Use Bioquell ProteQ for:
- Biopharmaceutical manufacturing areas
- GMP/GLP laboratories
- Biosafety labs
- Animal facilities
- Production areas such as RABS that are hard to clean manually
- Cleanrooms and anterooms
Why Choose Bioquell ProteQ
Effective
Uses Bioquell Hydrogen Peroxide Sterilant* to create hydrogen peroxide vapor that achieves a 6-log sporicidal kill on surfaces within an enclosed area.
*EPA Reg. No.
72372-1-86703
Integrated
One piece of equipment performs both vaporization and aeration. If needed, you can store additional aeration units within the unit. Use in existing facilities without complex modifications.

Easy to Use
Wireless connectivity allows for rapid setup and reduced downtime. Able to store and run 1000s of predefined cycles.

Compliant
Thermal and PDF printouts of key cycle data; 21 CFR Part 11-compliant audit trail available.
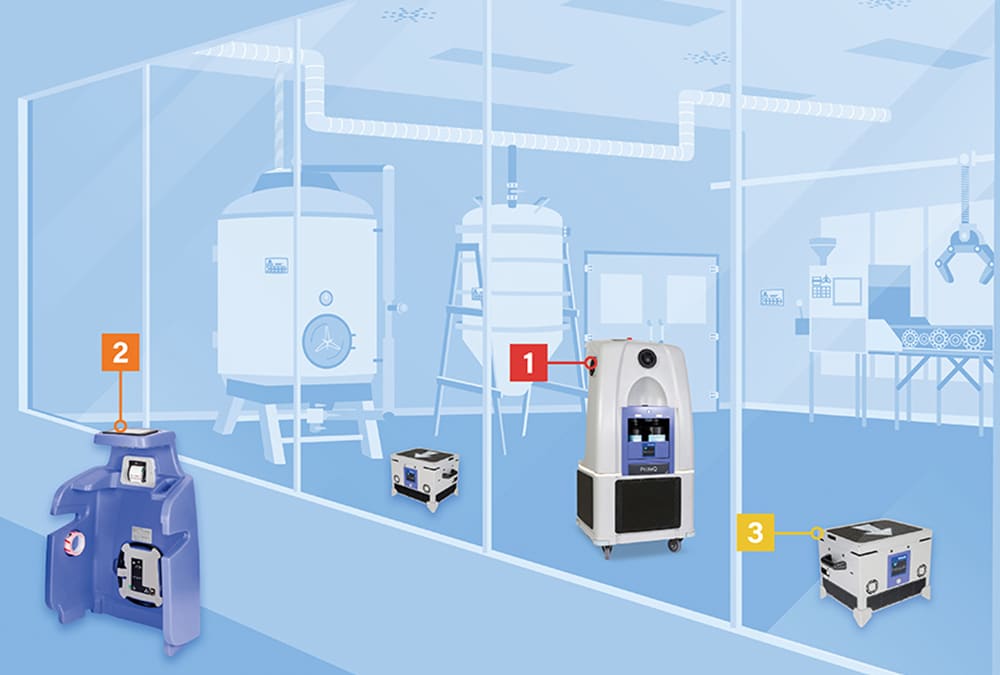
Bioquell ProteQ Components and Setup
Components:
- The main structure of the system contains:
- The hydrogen peroxide vapor distribution system for rooms up to 400 m³ (depending on configuration, load and environmental conditions).
- Dual-bottle module with RFID reading for proper loading, large volume treatment and key data collection.
- Built-in aeration, which is a standard feature in every Bioquell ProteQ unit.
- The wireless control module combines a thermal printer and a color touch screen with graphic interface.
- Additional aeration units are stored in the main structure of the system.
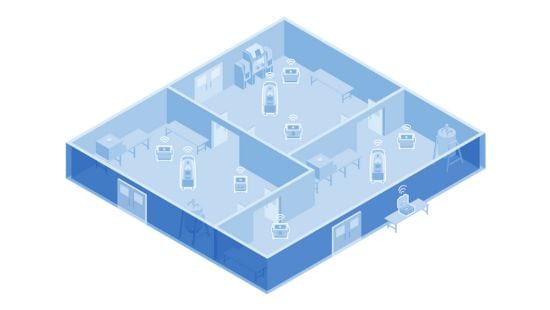
Multiple-System Setup
You can run up to three Bioquell ProteQ systems simultaneously from a single master controller, making larger spaces simple to treat. For zones requiring more than three systems, multiple master controllers can be used. Contact us for details.
Related Products and Accessories

Bioquell Hydrogen Peroxide Sterilant*
This is a 35% aqueous hydrogen peroxide solution used in Bioquell technology to generate hydrogen peroxide vapor, which achieves a 6-log sporicidal kill on exposed surfaces.
*EPA Reg. No. 72372-1-86703
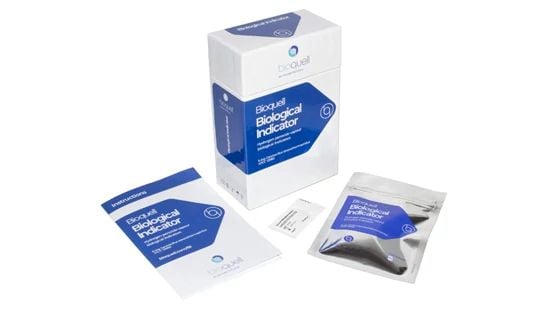
Bioquell Biological Indicators (BIs)
Our biological indicators are made from Geobacillus stearothermophilus endospores to help you validate a Bioquell cycle.
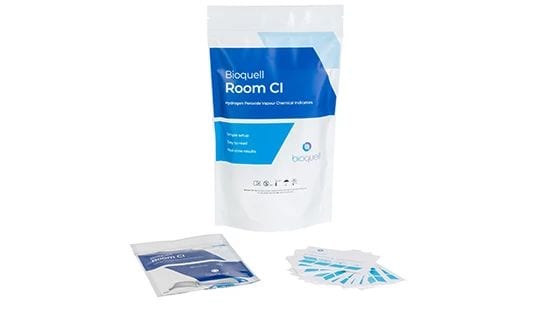
Bioquell Room Chemical Indicators (CIs)
Confirm that a Bioquell cycle has achieved the expected vapor distribution and exposure performance with these easy-to-read chemical indicators. A chemical strip reacts to a Bioquell cycle and changes color to correlate with the cycle’s expected performance. For use in rooms and enclosed areas 10m3 and larger.

Additional Aeration Units
More aeration units can be added to the Bioquell ProteQ to disperse vapor and provide further aeration, resulting in faster cycles. These units connect wirelessly with the system. Up to two aeration units can be stored within the frame of the Bioquell ProteQ, for ease of transport.

Low-Level Peroxide Sensor (XAM-5100)
This sensor helps you evaluate the levels of hydrogen peroxide remaining after completion of a cycle. It provides an added level of reassurance beyond the readings given by the Bioquell ProteQ system.

Validation
To meet regulatory requirements, you may need to deliver proof that your bio-decontamination solution performs as it should. Ecolab provides trained engineers and the validation documentation you need to comply with regulations.

Use Even on Sensitive Electronics and Equipment
Bioquell Hydrogen Peroxide Sterilant* vapor is tough on microbial surface contaminants, but has no ill effects on sensitive electronics. Unlike some spray-and-wipe methods, there are no additional steps to remove residue. When you need to bio-decontaminate spaces that contain hard-to-clean equipment, Bioquell is ideal. Before using Bioquell on your electronic equipment, check with the manufacturers to confirm compatibility.
*EPA Reg. No. 72372-1-86703
FAQs About Bioquell ProteQ
Can the Bioquell ProteQ control my HVAC?
Yes. The system can communicate with your BMS via volt-free contacts to indicate cycle progress. The BMS can then control your HVAC accordingly.
What if the wireless connection stops working in my building?
While extremely rare, if this situation occurs, we can supply a wireless range extender to ensure you're able to run cycles without interruption.


