
Bioquell Rapid Contamination Control Service
Do you need a reliable contamination control strategy in your lab or manufacturing facility, but lack the staff and equipment to carry it out? Bioquell Rapid Contamination Control Service from Ecolab is the answer.
This all-inclusive service handles every aspect, whether you want us to address a few small spaces or an entire building.
Benefits of Rapid Contamination Control Service
Bioquell Rapid Contamination Control Service is an all-in-one service that includes planning, coordination, setup, equipment, cycle verification, and detailed reporting.
Our skilled technicians work with you to customize a protocol that meets the requirements of your lab or production facility.
Rapid Contamination Control Service is ideal for:
- Emergency containment control
- Biosafety-level labs 2, 3 and 4
- Post-construction new-area commissioning
- Research areas
- Pharmaceutical production areas
- Annual shutdown for area maintenance
Why Choose Bioquell Rapid Contamination Control Service
Efficient
Our expert team provides quick setup and turnaround, including emergency response.
Reliable
Uses Bioquell Hydrogen Peroxide Sterilant to create hydrogen peroxide vapor that achieves a 6-log sporicidal reduction on surfaces within an enclosed area.
*EPA Reg. No.
72372-1-86703
Flexible
Custom treatment for any location size or project scope. Technicians are available on weekends, holidays and during off-peak hours to help you return to full operation as soon as possible.
Integrated
Design and flexibility blend into your existing process, preventive maintenance or emergency response plans.
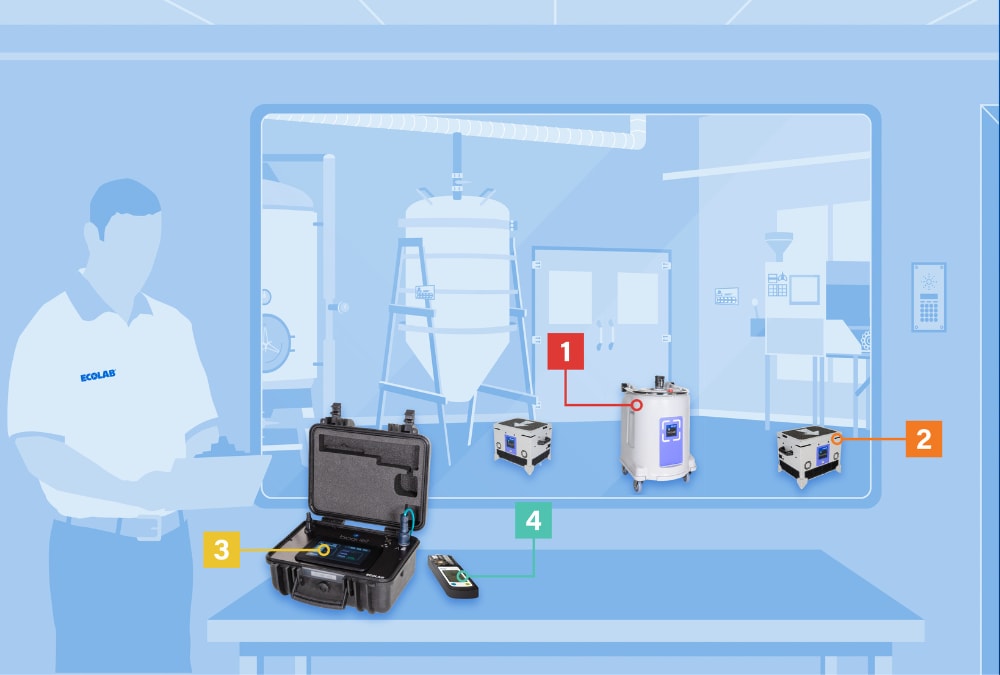
Components of Bioquell
Rapid Contamination Control Service
- Vaporizers are placed strategically to emit the hydrogen peroxide vapor. These can be networked with multiple generators to cover large areas.
- Aeration and distribution units help spread the hydrogen peroxide vapor at the start of the cycle and quickly remove it during the final stage.
- Control panel networks multiple vaporizers, environmental monitoring equipment (not pictured), and aeration units from a single device.
- Portable hydrogen peroxide vapor detectors are used to perform routine checks during a cycle, to ensure there are no leaks outside the treated area.
Watch the RBDS Process
Bioquell Business Continuity Service
In pharmaceutical and biologics manufacturing, a contamination event can lead to lost revenue, drug shortages for patients, and even a shutdown of your facility. The fastest way to get a contamination crisis under control is to prepare ahead of time.
Bioquell Business Continuity Service is a version of Bioquell RBDS. It’s designed for large organizations that want to be as ready as possible for contamination events, so they can react quickly.
Reporting and Confirmation

This service provides a complete report of the efforts undertaken in the selected area.
Each report includes the readings of our internally produced, high-quality biological and chemical indicators (BIs and CIs). Our BIs are made from Geobacillus stearothermophilus endospores. Bioquell CIs have specially formulated reactive dye to provide real-time indication.
Our reports also cover:
- Detailed cycle parameters and graphs
- Environmental conditions
- Incubation records of the biological indicators
- Maps showing the placement of all equipment and indicators
- Equipment calibration information
- Certificates of analysis for all products used
FAQs About Rapid Contamination Control Service
Do I have to close the whole building during the process?
Typically, you can continue to occupy adjacent work areas with no disruption. As part of the process, we use specialized sensors to ensure no hydrogen peroxide vapor is leaking into adjacent areas.
How long will it take for Bioquell to treat my area?
Each situation is unique. We will work with you to assess the area and provide a time estimate.
Can I leave my equipment in the area?
Yes, and we encourage you to do so. If you remove equipment before and then bring it back into the space, there's the risk of re-introducing contamination. Bioquell technology has been used on sensitive electronics with no ill effects.
I only need to treat equipment, such as the interior of my BSC, freeze drier, or isolator. Is this an option?
Yes. Please contact us for details.

About Hydrogen Peroxide Vapor
During a bio-decontamination cycle, Bioquell technology fills an enclosed area with vaporized Bioquell Hydrogen Peroxide Sterilant.* In just one cycle, this vapor provides a 6-log sporicidal kill on exposed surfaces. Unlike chemical sterilants that can leave toxic residues behind, hydrogen peroxide vapor breaks down into water and oxygen once the bio-decontamination cycle and aeration is complete.
*EPA Reg. No. 72372-1-86703


