Coronavirus: Healthcare Infection Control Precautions for COVID-19
Ecolab Disinfectants are Effective Against The COVID-19 Coronavirus.
Human coronaviruses1 are common throughout the world. Human coronaviruses commonly cause mild to moderate illness. Two newer human coronaviruses, MERS-CoV and SARS-CoV, have been known to cause severe illness. A novel coronavirus, SARS-CoV-2, which causes a respiratory infection called COVID-19, emerged in 2019. Chinese authorities identified the new coronavirus originating in Wuhan, China. Since its emergence, this virus has resulted in hundreds of thousands of confirmed cases globally. On March 11, 2020, the World Health Organization declared the situation a pandemic because the disease has spread over many countries and continents and affects a large percent of the population. In the United States, the Centers for Disease Control and Prevention (CDC) is working closely with state health departments and healthcare facilities on disease testing, surveillance, contact tracing, and interim guidance for clinicians on identifying, containing and treating COVID-19 infections.
What is COVID-19?
- Clinical signs and symptoms include fever and symptoms of lower respiratory illness (e.g., cough, shortness of breath).
- Early on, many patients in the outbreak in Wuhan, China reportedly had some link to a large seafood and animal market, suggesting animal-to-person spread. However, as the outbreak has evolved, community person-to-person spread is occurring. The situation with COVID-19 is evolving rapidly. While severe illness has been reported globally, other patients have had milder illness requiring no medical care. Person-to-person transmission has been reported in healthcare workers.
- People of all ages can be infected by COVID-19. Older people, and people with pre-existing medical conditions (such as asthma, diabetes, heart disease) appear to be more vulnerable to becoming severely ill with the virus.
What are the healthcare infection control precautions for COVID-19 as it relates to hand hygiene and environmental hygiene?3
Although the transmission dynamics have yet to be fully determined, CDC currently recommends a cautious approach to patients under investigation for COVID-19:
- Meticulous hand hygiene and environmental hygiene play a key role in these isolation precautions.
- Healthcare professionals should perform hand hygiene before and after all patient contact, contact with potentially infectious material, and before putting on and upon removal of PPE, including gloves. If hands are visibly soiled, use soap and water before returning to alcohol-based hand sanitizer.
- Healthcare facilities should ensure that hand hygiene supplies are readily available in every care location.
- Healthcare professionals must be cleared, trained and fit tested for respiratory protection device use, and educated, trained and have practiced appropriate PPE use including correct use to prevent contamination of skin, clothing or the environment, prior to caring for a patient with known or suspected cases of COVID-19.
- Healthcare professionals entering the patient room should use standard precautions, contact precautions, airborne precautions, and eye protection (e.g., goggles or a face shield). In situations where N95 respirators are in short supply, healthcare professionals may wear surgical masks for patient contact except in instances where aerosol-generating procedures are being performed, where an N95 must be worn.
- The EPA and the CDC recognize environmental surfaces as a vector for transmission of coronaviruses. The CDC has developed a hospital preparedness checklist4 which recommends that hospitals assess the effectiveness of environmental cleaning and consider providing refresher training on environmental hygiene best practices as outlined in the CDC Toolkit: Options for Evaluating Environmental Cleaning.5
- The CDC states that facilities must ensure that healthcare professionals receive job or task-specific education and training on transmission of infectious agents, including refresher training.
- Routine cleaning and disinfection are appropriate for COVID-19 in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed.
What hand hygiene products are effective against COVID-19?
Washing your hands often with soap and water is one of the best ways to avoid transmission of emerging pathogens. The World Health Organization recommends performing hand hygiene with soap and water or alcohol-based hand rub if soap and water are not available. The US Food and Drug Administration regulates claims on both medicated, antimicrobial soaps and on alcohol-based hand sanitizers. Claims related to efficacy against viruses are not allowed on any medicated, antimicrobial soaps nor on any alcohol-based hand sanitizers in the United States.
What disinfectants are effective against COVID-19?
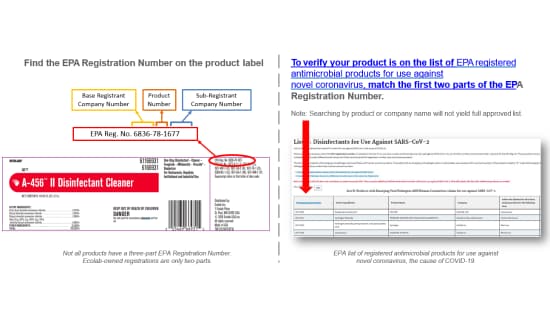
COVID-19 is caused by SARS-CoV-2. The EPA has developed the “Guidance to Registrants: Process for making claims against emerging viral pathogens not on EPA-registered disinfectant labels”.6 This document provides general guidance to disinfectant manufacturers and addresses public concerns on a process that can be used to identify effective disinfectants for use against emerging viral pathogens. It permits manufacturers to make limited claims about their product’s efficacy against such pathogens once the EPA confirms the product meets the eligibility criteria outlined in the guidance. In addition, the EPA has published List N: Disinfectants for Use Against SARS-CoV-2.7 See graphic below which shows how to know if a product is approved for use against COVID-19.
In addition, the table below lists Ecolab products that meet the criteria for claims against emerging viral pathogens and therefore can be used against COVID-19 when used in accordance with the directions for use against the listed supporting virus on hard, non-porous surfaces. Contact your Ecolab Account Executive for additional product information.
DAZO™ fluorescent marker is an objective method to evaluate the thoroughness of the cleaning process, rather than the presence of organic material or pathogens.5 Other environmental monitoring methods intended to measure organic load (e.g. ATP)6 or bacterial burden (e.g. bacterial cultures) do not detect viruses. Even with the best EVS staff in the world, without an effective environmental hygiene monitoring tool such as DAZO fluorescent marker, there is no way to determine the effectiveness of their cleaning practices.
HOW TO KNOW IF PRODUCTS ARE APPROVED FOR USE AGAINST CORONAVIRUS
| ECOLAB PRODUCT NAME | ITEM NUMBER (Package Size) | DILUTION RATE | CONTACT TIME | EPA REG. # | SUPPORTING VIRUS |
|---|---|---|---|---|---|
| A-456 II™ DISINFECTANT CLEANER | 6166931 (2x1.3L) |
0.5-1 oz/gal | 10 minutes | 6836-78-1677 | Norovirus (Feline calicivirus surrogate) |
| TB+IITMASEPTICARE | 6021521 (12x32oz) | Ready-to-use | 3 minutes | 1130-15-1677 | Rotavirus |
| NEUTRAL DISINFECTANT CLEANER | 6027314 (4x1gal) 6101205 (1x2.5gal) 6114541 (2x1.3L) 6100836 (50x1oz Pour Pak) |
0.5-1 oz/gal | 10 minutes | 47371-129-1677 | Adenovirus |
| OXYCIDE™ DAILY DISINFECTANT CLEANER | 6000189 (2x96oz) |
3 oz/gal | 3 minutes | 1677-237 | Norovirus (Feline calicivirus surrogate) |
| QUATERNARY DISINFECTANT CLEANER | 6063304 (4x1gal) |
0.5-1 oz/gal |
10 minutes |
6836-78-1677 | Norovirus (Feline calicivirus surrogate) |
| QUATERNARY DISINFECTANT WIPES |
6000166 (12x220ct) 6000169 (12x85ct) |
Ready-to-use | 2 minutes | 6836-372-1677 | Human Coronavirus |
| TB DISINFECTANT CLEANER RTU | 6143556 (12x32oz) |
Ready-to-use |
3 minutes |
1839-83-1677 | Poliovirus |
| VIRASEPT™ | 6002314 (12x32oz) |
Ready-to-use |
4 minutes |
1677-226 | Rhinovirus |
For More Information:
- World Health Organization, Coronavirus: www.who.int/health-topics/coronavirus
- World Health Organization, Coronavirus disease (COVID-19) advice for the public: Myth busters
- Centers for Disease Control and Prevention, Coronavirus Summary
References:
- Centers for Disease Control and Prevention, Coronavirus Summary. www.cdc.gov/coronavirus/2019-ncov/
- Centers for Disease Control and Prevention, 2019 Novel Coronavirus.
- Centers for Disease Control and Prevention, Interim Healthcare Infection Prevention and Control Recommendations for Patients Under Investigation for 2019 Novel Coronavirus
- Centers for Disease Control and Prevention, Hospital Preparedness Checklist for Suspected or Confirmed 2019-nCoV Patients. www.cdc.gov/coronavirus/2019-ncov/hcp/hcp-hospital-checklist.html
- Centers for Disease Control and Prevention, Options for Evaluating Environmental Cleaning. www.cdc.gov/HAI/toolkits/Evaluating-Environmental-Cleaning.html
- Environmental Protection Agency, Emerging Viral Pathogen Guidance for Antimicrobial Pesticides. www.epa.gov/pesticide-registration/emerging-viral-pathogen-guidance-antimicrobial-pesticides
- Environmental Protection Agency, List N: Disinfectants for Use Against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19



